Phase Diagrams
Textbook Readings
11.8: Phase Diagrams
Course Lectures
11.4 pdf Video Phase Diagrams
Homework Problems
Refer to
the phase
diagram at right when
answering questions 76.1 - 76.6
answering questions 76.1 - 76.6
76.1 What are the physcal states corresponding
to regions i. , ii. , iii. and iv.
76.2 What is carbon dioxide's physical state
at room temperature (~23oC) and
atmospheric pressure (1 atm)?
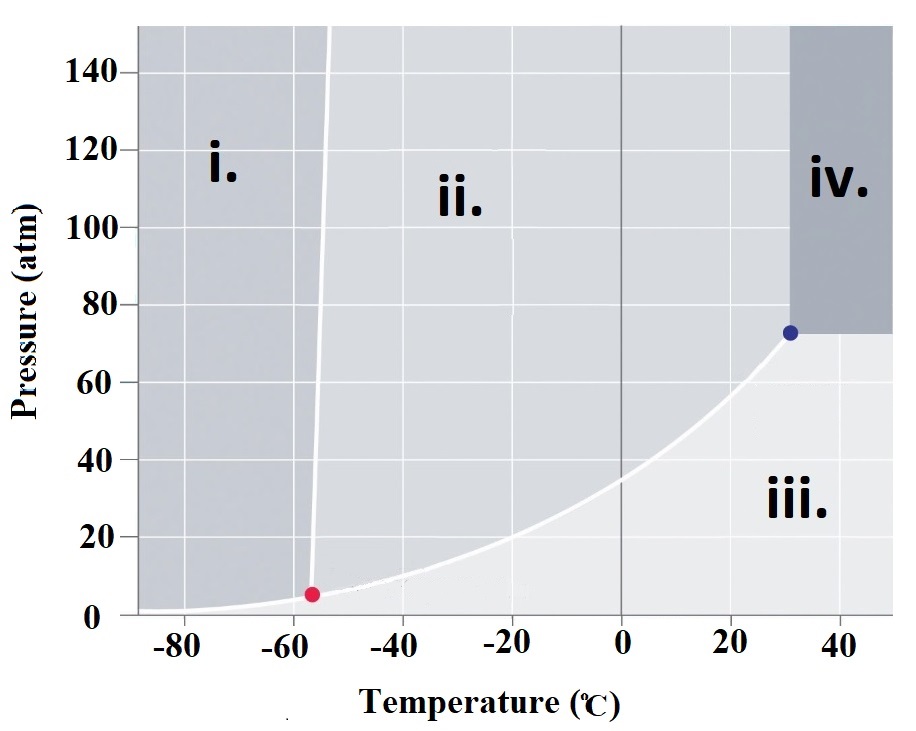
corresponding to CO2's triple point?
76.4 What is the physcial state of CO2 at a pressure of 80 atm and a temperature of 40oC?
76.5 A CO2 sample at a pressure of 60 atm and a temperature of -20oC is warmed to a
temperature of 40oC while keeping the pressure constant.
What is the order of phase changes that occur along this path?
76.6 Consider a CO2 sample at 140 atm and -58oC. If the pressure is gradually decreased to
0.1 atm, what phase changes are observed?
76.7 What is a supercritical fluid?
76.8 Examine water's phase diagram available here. What phase changes occur as
you go from point A to point B. What is so unusual about the order of these phase changes?
Click and drag the region below for correct answers
76.1 i. Solid ii. Liquid iii. Gas iv. Supercritical fluid
76.2 Click here: Gas
76.3 Click here: The triple point is that set of conditions that CO2 can exist simultaneously as a solid,
liquid and gas. For CO2, this corresponds to T = -56.57 oC and P = 5.11 atm.
76.4 Click here: Supercritical Fluid
76.5 Click here: Liquid -> gas
76.6. Click here: Decreasing the pressure eventually leads to the solid converting into a liquid.
Finally, the liquid changes into a gas.
.
76.7. A supercritical fluid exists at pressures and temperatures where liquid and gas phases become
indistinguishable. View this video link for more information on supercritical fluids.
76.8 As pressure is increased, the density of the material must increase.
This water system begins as a gas. As pressure is increased, the gas is converted into a solid.
As even more pressure is applied, the solid is converted into a liquid.
In other words, solid water (a.k.a. ice) is more dense than water vapor. However,
ice is less dense than liquid water.
For most materials, the solid phase is the most dense. However, in the case of water, the
liquid phase is the most dense!